Chemistry, Manufacturing and Controls Strategy Meeting | Boston
Le Meridien Boston Cambridge Hotel 20 Sidney St, Cambridge, MA, United StatesLondon Labs Live 2025
ExCel London , United KingdomFuture Labs Live 2025
Congress Center Basel Basel, SwitzerlandChallenges call for improvement and it’s never too late to improve. In most clinical trials even though CRF designing happens at foremost step, the study protocol amendments demand for inevitable changes or updates in currently ongoing studies CRF Designs. As everyone knows, Rave EDC provides a platform for database update. Updating an ongoing study database is not a stopping point anymore as they can now be performed in ease using this platform and is worth learning for.
Can you think “Medicine without Salt” or “Research without Quality”? And over that, if there is someone to inspect you for the quality and ethics of your study! Don't worry, Zifo has a back for you with the best combination of quality and success!! Whether you're a researcher or sponsor, compliance with good clinical practices and use of the latest guidelines is essential for ensuring the quality and safety of research. Within the same hand, the FDA identifies potential issues early on and prevent further problems, fostering a culture of benefiting public health program with Bioresearch Monitoring (BIMO).
Hop on the wagon and get to know the story on Medidata RAVE EDC version updates countermeasures! Recurrent RAVE version releases are made to standardize EDC handling in recent years. Often these changes are a hard nut to crack. Hang in there…We've got your back. We shed light on the key hurdles and our methodology to overcome those challenges for smoothening enhancement. Two heads are always better than one.
Listen to Raj and Arno speaking about Zifo's acquisition of beyontics, the background of the two companies' histories and relationships, and what customers can expect in the future.
Industry leaders discussed their experiences & experiments.We have evolved and adapted to the recent pandemic and so has CDISC! We are working our way through these tough times and trying to ease the process of understanding the changes made to the latest SDTM version 3.4 this November 2021. To help us out during this pandemic and the way these studies are being conducted recently, CDISC has provided us with more clarification on the existing grey areas from the previous version.
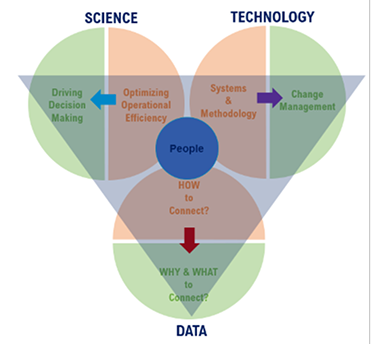
Influencing factors and strategies to consider for an ELN upgrade.
Welcome to the new normal. For our pan-India virtual study on the global pandemic, we leveraged social media to help us reach the right subjects. Listen to how we built trust through social media for our decentralized trial.
An analysis of warning letters and notices reveals that most R&D labs have insufficient measures to ensure Data Integrity compliance. In this video we discuss cost-effective ways to perform Data Integrity Remediation at your organization.
Being part of the clinical research industry, we are confronted by the COVID-19 situation to demonstrate leadership and guide the world to safety. We need to ensure that research continues to happen even amidst this question of existence. Here we share our thoughts on the impact of COVID-19 on existing clinical trials.
CDISC released ADaM IG version in last quarter of 2019. Do you want to know what has been addressed but don't have the time to go through the document? No worries. Our CDISC Experts give insights to the new version of ADaM IG in this webinar.
It's high time to configure library management system as a foreground service from CRF to TLF generation. Generally, we may work in a great number of simultaneously ongoing clinical trials, and most of the designs require the same set of standards. There are so many ways to configure an assortment based on requirements. But how do we ensure that the assortment is efficient and governed?
Did you know there are clinical trials that actually govern the studies of multiple drugs for the same indication or the same drug tested for multiple indications - all within ONE protocol? Welcome to the world of Master Protocols! In this video, our experts dive into the risks and opportunities associated with it, and discuss what to keep in mind while planning such studies.