Life Sciences Companies are under growing pressure and need to
- Unlock Efficiency : Easy access to scientific data. 70% companies report that accessing scientific data for AI initiatives is difficult today
- Navigate the Data Deluge : AI can provide the essential tools to extract actionable intelligence, and drive data-driven decision-making across the enterprise
- Drive Innovation : 35% of companies say AI is not just about automation; it’s a powerful catalyst for innovation
- No fluff : Designed to help science-focused organizations adopt AI effectively
- Practical AI3 Zifo’s AI framework is focused on real world ROI through AI adoption
- Practical Ai Now : Blueprint Hub for developing 100+ "Blueprints" focused on immediate value
- Practical Ai Next : Client Customised AI Process map for Incremental Improvements leading to Compounding Benefits
- Practical Ai Future : Setting the stage for long term Strategic Competitive Advantage through AI
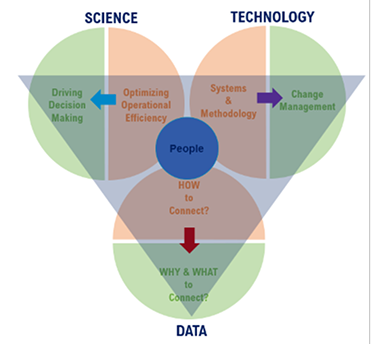
Zifo’s Practical AI3 framework:
Got an AI idea? Let’s Collaborate!
Completed Blueprints
AI Based Antibody Design
Accelerating Antibody Design with Zifo’s AI-powered Protein Language Models
AI Based Conversation Agent for Scientists
Redefine Scientist Experience through AI Guided, Voice Based Contemporaneous Data Capture Agent
AI to Reduce Assay Review Cycle Time
Zifo’s AI Solution speeds up deviation detection, ensures compliance, and validates hypotheses
AI Solution to Accelerate Computer System Validation
AI-driven validation streamlines processes, boosts accuracy, and delivers upto 75%-time savings
No-Code, AI Conversational Dashboard
Zifo’s AI-driven Conversational Interface to Visualize and Analyze Data for Instant Insights
AI Based CoA Automation
AI Solution to validate CoA data, Maintain Compliance and Accelerate Quality processes
AI Powered Audit Trail Review
Automatically generating checklists and parsing audit logs can streamline the audit trail process in regulated systems, ensuring data integrity, traceability, and GxP compliance.
AI Powered Data Migration
In regulated industries such as Biopharma, AI-enabled data migration solution automates complex tasks while ensuring validated data transfer, which is critical for compliance and innovation.
AI Powered Document Authoring
Infusing AI into the manual and time-consuming process of creating complex regulatory submissions – such as Clinical Study Reports (CSRs), Investigator Brochures, and CMC submissions – cuts first-draft timelines from days to hours.
AI Powered SOP Alignment
An AI-driven compliance assistant that compares your SOPs with FDA 483 findings, flags gaps before audits, and even suggests ready-to-use fixes - helping scientific organizations stay inspection-ready and ahead of risk.
AI Powered Document Creation
Manual documentation is a slow, tedious, and inconsistent process. Zifo solves this by using AI to analyze video content, automatically identifying key features and workflows to generate a structured, readable user manual in minutes, not days.
AI Powered 21 CFR Part 11 Solution
Due to the time-consuming, inconsistent, and error-prone nature of traditional 21 CFR Part 11 compliance assessments, Zifo’s AI-powered solution transforms weeks of manual expert review into hours of analysis, reducing regulatory risk and accelerating market delivery for biopharma products.
AI Powered USDM Design
Accelerate clinical trial design and execution by transforming all study protocols into living, machine-actionable assets mapped to the Unified Study Design Model (USDM).
AI Powered BC Creation
Manual, fragmented BC curation is inefficient, error-prone, and hinders data reuse. Zifo’s AI/LLM solution offers the opportunity to standardize, map, and create reusable BC assets, ensuring consistency and massive efficiency gains.
AI Powered Clinical Data Lineage
Zifo's AI Study Lineage automatically extracts and maps Biomedical Concepts (BCs) across protocols, CRFs, and SDTM. This creates semantic consistency and full traceability, transforming fragmented clinical documentation into reusable, high-quality data assets, resulting in accelerated study setup, reduced errors, and regulatory compliance.
Project Workbench
A chat interface with capabilities like web search, deep research and information retrieval from files stored in the workspace.
Zifo's Ontology Assistant
An ontology creation assistant that reuses information from public ontologies to create an ontology for the specific use-case. This automates the otherwise manual, time intensive ontology development steps, thereby accelerating creation of new Ontologies.
EHS Policy Update Engine
An engine that proactively recommends updates to organizational EHS policies to align with evolving regulatory expectations and compliance trends.
Data curation
Data extraction from FDA labels, enrichment of extracted data from sources like DrugBank, ChEMBL, UniProt, NCBI, and Ensembl along with mappings to relevant Ontology classes for creation of a Knowledge Graph.
GxP User Management
AI-powered user management engine that automates training validation, approval workflows, and escalations—accelerating compliant access provisioning while reducing governance overhead and onboarding delays.
Scheduling support
AI augmented scheduling of tests for samples using Copilot studio.
Experiment Insight generation
AI assisted insight generation from the experiment conclusions recorded by scientists. This enables easy analytics and quick decisioning by leveraging the huge volumes of data generated during research.
In Progress Blueprints
AI Powered Ontology Assistant
Create scalable, interoperable ontologies using Zifo’s AI-enabled Ontology Assistant.
More details coming soon...
AI Augmented EHS assistant
An AI-driven compliance assistant that compares an existing EHS policy with the latest updates in the regulatory guidelines and suggests modifications to help scientific organizations stay better compliant and inspection-ready.
More details coming soon...
Application Support Framework
An AI-powered unified console that optimizes application support through smart issue analysis, guided troubleshooting, and integrated account and task management.
More details coming soon...
Agentic LIMS DevOps
Natural language business requirement analysis to generate a configuration guide for LIMS (Sapio, Sample Manager, Labvantage).
More details coming soon...
QC Method Harmonization Solution
A harmonization solution that compares multiple QC method-execution variants and overlays it with custom business rules to generate a harmonized output. This can greatly help organizations quickly adopt digitization initiatives and streamline processes across multiple sites.
More details coming soon...
SOP update assistant
A multi-agent orchestration engine that interprets a desired future state, compares it with current state and identifies procedural gaps in current SOPs to recommend updates aligned with the proposed future state.
More details coming soon...
SDTM Automation
Leverages Agentic AI to reduce the workload of CDISC Developers by Generating SDTM conversion R Programs.
More details coming soon...
LCS
An aid to support seamless OS upgrades in laboratory environments. It offers structured AI-powered compatibility analysis of the myriad software packages, enabling faster, more reliable OS migration decisions.
More details coming soon...
ROS scorecard
A framework for ranking of Synthesis routes for Drug Substances based on customizable business logic. The engine ranks multiple ROS generated by different tools and presents them for easy consumption by chemists.
More details coming soon...
Data Verification
A framework to help establish the provenance of data when data moves across multiple platforms like ELN > snowflake > JMP.
More details coming soon...
Database Summarization
An agentic summarization and validation pipeline to generate key metrics and specific insights from data.
More details coming soon...
Margin Analysis
Evidence extraction and reasoning pipeline that extracts key information from regulatory documents to computes equivalence or non-inferiority margins to aid quick and intelligent study design creation.
More details coming soon...
Data Modelling Assistant
An engine to speed up data model creation for a specified use case.
More details coming soon...
Edit Check Automation
Automated generation of JavaScript codes aligned with Viedoc 4 EDC standards for clinical trials, enabling faster development and regulatory-compliant data capture.
More details coming soon...
Product Portfolio Support Engine
A product portfolio decision support engine powered by information on innovator patent expiry information and intelligence gained from market research to guide planning for generic entry.
More details coming soon...
Empower peak integration
AI assisted chromatographic peak integration resulting in faster processing due to minimal human intervention.
More details coming soon...