
On the other day, I was speaking to my friend who works in process development for a chip manufacturer in New Mexico. Being a jolly person in general, hearing him speak this time around was miserable.
He described how his organization was re-purposing its manufacturing line and shelved his role as non-essential for the time being. At the end of our conversation (in which I was only a patient listener), referring to me, he remarked how working in scientific research at this time was a boon.
I would have corrected him, but the poor fellow was already beaten worse. Moreover, what he said was only the common (mis)perception I was encountering all around. Though it is true that biotech and healthcare stocks are expected to perform better than those of other sectors in the ongoing circumstances, the reality is not all biotech stocks across the industry are performing well.
For instance, some clinical-stage biotech companies are seeing delayed drug pipelines due to quarantine measures and this is keeping their stocks extremely volatile.

As a R&D informatics solutions provider to a wide range of scientific organizations across the world, we at Zifo are at a vantage point to observe and understand how the life sciences industry is working to address this pandemic and how the pandemic has affected Scientists, Researchers, Vendors focused on Drug Discovery & Development.
The following are few intimate observations that try to capture the ground situation in large Pharma companies and small Biotechs.
#WFH
Securing employee and patient safety is being the priority now. As a side effect, most of our pharma and biotech customers’ laboratories are either shut or operated with bare minimum occupancy. This has plunged R&D plans and timelines into uncertainty.
We regularly partner with and provide services to a scientific informatics solution provider based in Japan. They are committed to personally implementing completed projects at their customers’ sites. This however is not going to be a possibility in the near future. Because of this, the projects that we completed a few months back have not been officially closed.
If we ignore such hiccups, there are a few turns that have marked improvements and innovative workarounds to note. For instance,
Moving the goalposts
Reprioritization is essential when times are challenging and unpredictable.
One of Zifo’s partners is a research lab operating under the government of India. They have now repurposed their genomics lab to a PCR-RT testing lab for COVID-19.
Similarly, one of our UK-based customers has shifted their project leads to work with Cambridge University to build an emergency COVID-19 testing laboratory. This temporary shift has also freed up laboratory research funds which our customer has now reallocated for other projects that could be done remotely in the meantime, including business consulting.
Another curious case of facing the heat and cold at once was seen in our customer who is a global leader in laboratory instruments, consumables, and software.
They cater to a range of laboratories from pharmaceutical and chemical to food and beverages and oil and natural gas. Because each of these sectors have reacted differently and by varying degrees to the pandemic, our customer is relooking at their portfolio and conducting weekly evaluations to ensure their teams are operational and sufficiently allocated.
One of their customers, a Dutch brewing company, is seeing extensive demand for beer and is looking to revitalize their manufacturing landscape with our customer’s scientific informatics tools to meet the demand. But another of their customers who comes from the oil and natural gas industry has pulled the plug on many agreed deals.

A positive mentality to change management can go a long way, neatly exemplified by an American life sciences company and a Swiss company we work with.
At the German site of the American company, production of medical devices and equipment is in full swing. Team members who are not a part of this are working from home and identifying SOPs/forms that have not yet been automated (paper-on-glass) and passing onto us for workflow or templates development.
Interestingly, they are doing this by creating detailed Visio diagrams of all laboratory SOPs and connecting them to arrive at the overall process flow of all their German laboratories. This has helped them in easier identification and tracking of automated and non-automated processes.
Scientists at the Swiss company are avoiding isolation blues by using their time to test and enhance the prepared automation workflows that they could not work on erstwhile. Our team recently experienced a surge of automation requests and enhancements with enthusiastic turnaround timelines.
One pixel at a time
An interesting fact: Netflix has more than doubled the number of new subscribers from February to April as more people were settling down to work, study, or holiday at home. Confined, people who did not need Netflix earlier were adopting digital entertainment as a solution to lift up their spirits.
We also saw how work-from-home was driving digital adoption in terms of executing and exchanging contracts and records digitally.
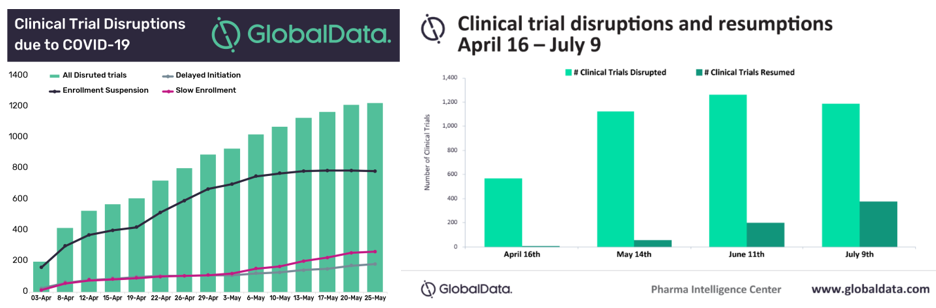
Similarly, the need to work remotely and the restrictions on face-to-face contact have compelled physicians in charge of disrupted clinical trials to adopt more digital platforms – a move that industry observers thought would take years combined with immense persuasion and investments. Truly, necessity is the mother of all invention.
(For trial sponsors who are in the grey area regarding the general continuity of clinical trials. We have come up with an FAQ that deals with how disruptions in clinical trials can be handled according to industry guidelines.)
We recently designed a virtual trial simulation to study the challenges and possibilities in a decentralized digital clinical trial.
The research is a registry study running in South America with 50 subjects and 2 sites. The subjects come to the site once every month and provide data and also blood samples.
In the next stage, the pandemic affects this routine and subjects are not able to travel to the sites and the sites are also not able to focus on clinical trials due to other priorities. The study is due to get over in 3 months and they are thinking of closing down the study as the last option.
At this stage, Zifo evaluates options and comes up with a solution where the CRFs are built in ePRO that can be accessed via mobile phones. The data part is taken care of, but blood sample is a showstopper. Luckily, this study does not need a centralized lab and so we tie up with a lab vendor around the sites who could do home visits and collect blood samples.
However, we cannot rely on the subjects to enter data and there are issues with internet connection. So we configure the mobile app to enable offline data entry. We train the lab vendor to collect data in offline mode so that it will sync with the database once it latches on to an active connection.
For years, we have been championing the use of digital technologies including AI, ML, IoT, etc., to accelerate R&D processes, ensure compliance and reduce costs, and to see digital adoption gaining favour now is great news.
COVID-19 has surely opened Pandora’s box. We have seen both setbacks and leaps, blessings and curses in the scientific research industry. Being an important stakeholder in global R&D, we are adapting ourselves to this new normal. We are redefining our approach and methodologies to be flexible to support R&D organizations that are taking cautious, slow and steady steps as well as those that want to make full use of this situation to overhaul their processes and informatics landscapes.
(On that note, we are offering our services free of cost for COVID-19 programs and will ensure that these projects will be handled on the highest priority. You can read about this and contact us here.)
While these turbulent times are impacting different people and organizations differently, there is no doubt that together we will cross this period emerging stronger.


