Ever since the announcement of the novel coronavirus as a pandemic, all that I got to hear was about the significant toll of the COVID-19 outbreak all over the world with positive cases expanding more than 3.6 million and 2,50,000 deaths as of May 5, 2020.
If there was something that seemed to prevail among all societal groups irrespective of any discrimination, then it was fear, anxiety and uncertainty and of course I was no exception. I turned more anxious when the health crisis started to take shape as a social and economic crisis as well, which led around 175 countries across the globe announce a nation-wide lockdown to mitigate the spread.
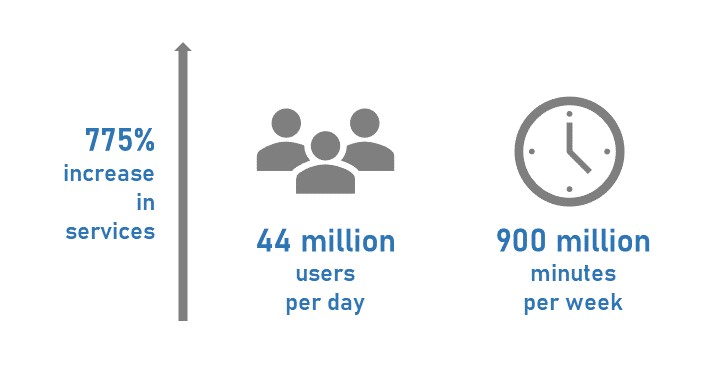
Running across headlines on how global trade of essential commodities like oil were drastically affected, leading to the sharp fall of prices, sounded like a warning towards economic recession. Working in the Scientific RnD Informatics sector and having crossed almost 50 days of remote work lifestyle, made me curious to know as to how sectors like us are coping up with the shutdown and what is awaiting us in the post pandemic run. Surprisingly, I happened to witness a spurt of growth in the business of cloud technology providers such as Amazon, Microsoft and Google especially during these times and analysis show that they are expected to emerge as the champions at the end of this phase for several reasons.
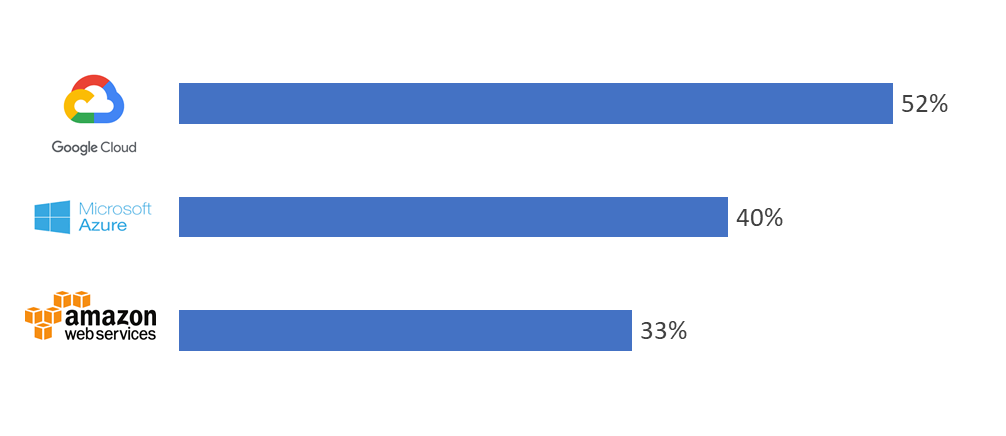
Similarly, AWS has put in place several disaster recovery policies and provides confidence to handle the spike in demands during the period.
Also, looking back at what can be learnt from earlier times during similar pandemics, it is evident that pharma and healthcare sectors were those who continued to operate with even more rigor. Returning to normal conditions has happened only with the discovery of a vaccine. Unlike any other companies which proceed with inventing and selling better versions of their products, pharmaceutical and biotech companies undergo a lot more of procedures and trials to achieve the same with respect to a drug/vaccine, since they are bound by a set of regulatory and compliance guidelines.
The drug development process in general involves several phases of experimental studies and clinical trials which require diverse data collection, processing and analysis of huge chunks of data in collaboration with laboratories across the globe.
As of today, with the list of completed and ongoing COVID-19 studies registered in the WHO database amounts up to 1,042 and “time” being the essence of success in the pharma & biotech sectors, a solution is required to accelerate the process with limited/no movement.
When I was chatting with our Digital Transformation, Cloud Services and HPC teams, they said cloud computing seems to have received the attention of quite a few pharma and biotech companies as an avid jump into digital transformation and cloud migration might help them in terms of recording, storage, processing and analysis of humongous data from anywhere in the world. Moreover, adopting HPC based cloud services might provide a whole lot of advantage in terms of faster computation and simulation resulting in improved performance. Also, cloud services over time have come up with various features to be secure and compliant with the regulatory guidelines.
With all these considered, a huge surge in the demand for cloud services are expected at the end of the COVID -19 pandemic. Would it make it an essential infrastructure?