Blueprint For Pharma Success: A Strategic Guide To Informatics Vendor Evaluation

Image reference: / source
In today’s rapidly evolving pharmaceutical landscape, data is the new drug. From early discovery to global commercialization, informatics systems have become the backbone of innovation, regulatory compliance, and operational excellence.
Yet, with a crowded vendor marketplace and increasing regulatory scrutiny, selecting the right software partner is no longer optional, it’s mission-critical. According to industry benchmarks, 70% of clinical trial delays stem from vendor misalignment, leading to 20–30% budget overruns. Moreover, failure to comply with data protection regulations such as HIPAA or GDPR – often due to vendor-related shortcomings – can result in penalties ranging from $100,000 to several million dollars.
In an environment defined by stringent regulations, a relentless pursuit of quality, and the demand for seamless digital integration, effective vendor evaluation is not just a best practice – it’s the foundation of compliance, innovation, and competitive advantage. Let’s explore the core principles, a comprehensive framework, and practical methodologies to empower your vendor selection process.
Core Principles of Vendor Evaluation
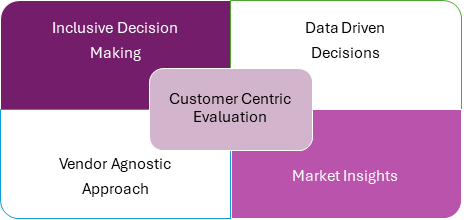
1. Inclusive Decision-Making: Bringing Everyone on Board
Effective vendor evaluation starts with collaboration. By involving business, IT, and other stakeholders early in the process, organizations ensure that decisions are well-rounded and widely supported. This inclusive approach fosters ownership and eases the transition to new vendor relationships.
2. Data-Driven Decisions: Let Facts Lead
Decisions grounded in data are more credible and defensible. A structured methodology provides leadership with evidence-based insights, enabling clear, objective, and confident decision-making.
3. Vendor-Agnostic Approach: Level Playing Field
Neutrality is key. A vendor-agnostic framework ensures all vendors are evaluated fairly, based on performance and fit-not on brand familiarity or bias. This encourages competition and innovation, helping organizations choose the best partner for their needs.
4. Customer-Centric Evaluation: Focus on What Matters
Every customer is unique. By understanding and prioritizing what matters most-be it scientific capabilities, regulatory compliance, or partnership potential-organizations can align vendor selection with their strategic goals.
5. Market Insights: Stay Ahead of the Curve
Incorporating current industry trends and market intelligence into the evaluation process ensures that selected vendors are not only capable today but also future-ready. This foresight supports long-term success and adaptability.
Vendor Evaluation Framework
The vendor evaluation framework aids in selecting potential vendors through a structured format. This framework relies on a set of standard criteria to assess the suitability of prospective vendors and the associated risks. There is no single vendor evaluation method that covers every circumstance. The approach may vary depending on the company’s maturity stage and size etc.
Preparation:
Business Drivers
- Business Value: Benefits a project brings, including financial gains, market share growth, and customer satisfaction.
- Success Criteria: Metrics to determine if goals are met, such as KPIs, milestones, and deliverables.
Complexity: Assessment of project difficulty, influenced by stakeholders, technical challenges, and interdependencies. - Maturity: Level of development and sophistication of the organization’s processes and capabilities.
Readiness: Organization’s preparedness, including resource availability and team readiness.
Strategic Direction
- Product Pipeline: Planned sequence of products or services to be developed and brought to market.
Delivery Model: Approach to delivering products or services, including traditional and modern methods. - Disruptive Trends: Emerging trends and technologies that could significantly impact the industry and competitive position.
Opportunities
- Process: Areas for process improvement to increase efficiency, reduce costs, and enhance quality.
- People: Enhancing skills, collaboration, and work culture to drive innovation and performance.
Platform: Technological infrastructure and tools, including adopting innovative technologies and upgrading systems.
Prioritization
- Future State Capabilities: This involves identifying and prioritizing the capabilities that the organization needs to develop to achieve its strategic goals.
- Prioritization ensures that resources are allocated to the most critical areas that will drive the organization’s success.
Comprehensive Approach
Function Evaluation
When evaluating vendors, it’s crucial to assess their knowledge of the domain, business processes, emerging needs, and industry best practices. Understanding the product functions is another key aspect of function evaluation. This includes knowing what features are included “out of the box,” what can be configured to meet specific needs, and what requires customization. A robust solution should be capable of delivering a streamlined user experience, ensuring that the end-users can efficiently and effectively utilize the product.
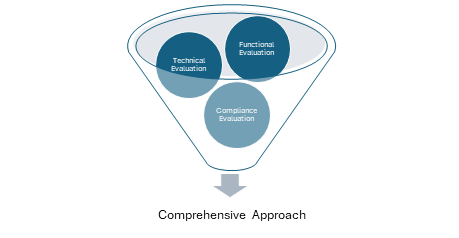
Technical Evaluation
Technical evaluation involves a thorough understanding of the vendor’s technology stack, infrastructure requirements, and performance benchmarks. Evaluating the user interface, integration APIs, data models, and dashboards is also essential to ensure that the solution is user-friendly and can seamlessly integrate with existing systems.
Additionally, it’s important to assess the hierarchy, security, audit, archiving, data integrity, and FAIR (Findable, Accessible, Interoperable, and Reusable) capabilities of the vendor’s solution.
Compliance Evaluation
Compliance evaluation focuses on understanding the vendor’s adherence to regulatory requirements, such as electronic signature and 21 CFR Part 11 compliance. This ensures that the vendor’s solution meets the necessary legal and regulatory standards for electronic records and signatures. Evaluating record retention, electronic records, and data archival/migration policies is also crucial to ensure that the vendor can securely manage and preserve data over time.
Furthermore, it’s important to assess the audit trail and data privacy features of the vendor’s system. A solution compliant with 21 CFR Part 11 requirements should provide a comprehensive audit trail, ensuring that all actions are recorded and traceable. Data privacy measures should also be in place to protect sensitive information and ensure compliance with data protection regulations.
Supplier Evaluation
This pertains to the vendor company’s operations, licensing and subscription models, support and professional services, and general inquiries including their site presence. Occasionally, it involves IT-related aspects.
Conclusion: Building a Resilient Vendor Network
Effective vendor evaluation in the pharmaceutical industry is essential for ensuring quality, regulatory compliance, and the consistent delivery of safe and effective medicines. A structured approach encompassing regulatory adherence, quality assurance, risk management, and ongoing performance monitoring enables companies to build and keep a reliable vendor network while mitigating risks and optimizing costs.
Looking ahead, artificial intelligence (AI) is set to revolutionize this process by automating documentation, analyzing vast datasets for compliance and performance trends, and uncovering hidden risks across vendor portfolios. By integrating AI into vendor evaluation workflows, pharmaceutical companies can enhance efficiency, improve decision-making, and unlock new possibilities for smarter, more proactive vendor management.
Reference:
1. Budish, Eric, Benjamin N. Roin, and Heidi Williams. 2015. “Do Firms Underinvest in Long-Term Research? Evidence from Cancer Clinical Trials.” American Economic Review,105(7):2044–85 – https://pubs.aeaweb.org/doi/pdfplus/10.1257/aer.20131176
2. Matthey, M.A., Hollis, A. Pull me – push you? The disparate financing mechanisms of drug research in global health. Global Health 20, 14 (2024): https://globalizationandhealth.biomedcentral.com/articles/10.1186/s12992-024-01019-x
3. Day of Delay White Paper Final
4. Smith, Z.P., DiMasi, J.A. & Getz, K.A. New Estimates on the Cost of a Delay Day in Drug Development. Ther Innov Regul Sci 58, 855–862 (2024). https://doi.org/10.1007/s43441-024-00667-w