Modeling and Simulation (M&S) tools have become essential to modern pharmaceutical R&D. From simulating protein-ligand interactions to optimizing dosing strategies, M&S supports faster, more informed decisions across every stage of drug development.
Yet in many pharma organizations, M&S capabilities are still fragmented, siloed across departments, built on redundant infrastructure, and supported by duplicated licenses. The result? Rising IT costs, poor reproducibility and missed opportunities for collaboration.
It’s time for a change.
How M&S tools power the drug development lifecycle
Modeling and Simulation tools enable scientists to test hypotheses, simulate drug behavior, and predict clinical outcomes, often before stepping into a lab. They reduce experimental burden, accelerate decision-making, and improve translatability across discovery and development. Multiple specialized tools are used at different stages, from structural modeling in early discovery to PK/PD and QSP simulations in clinical development.
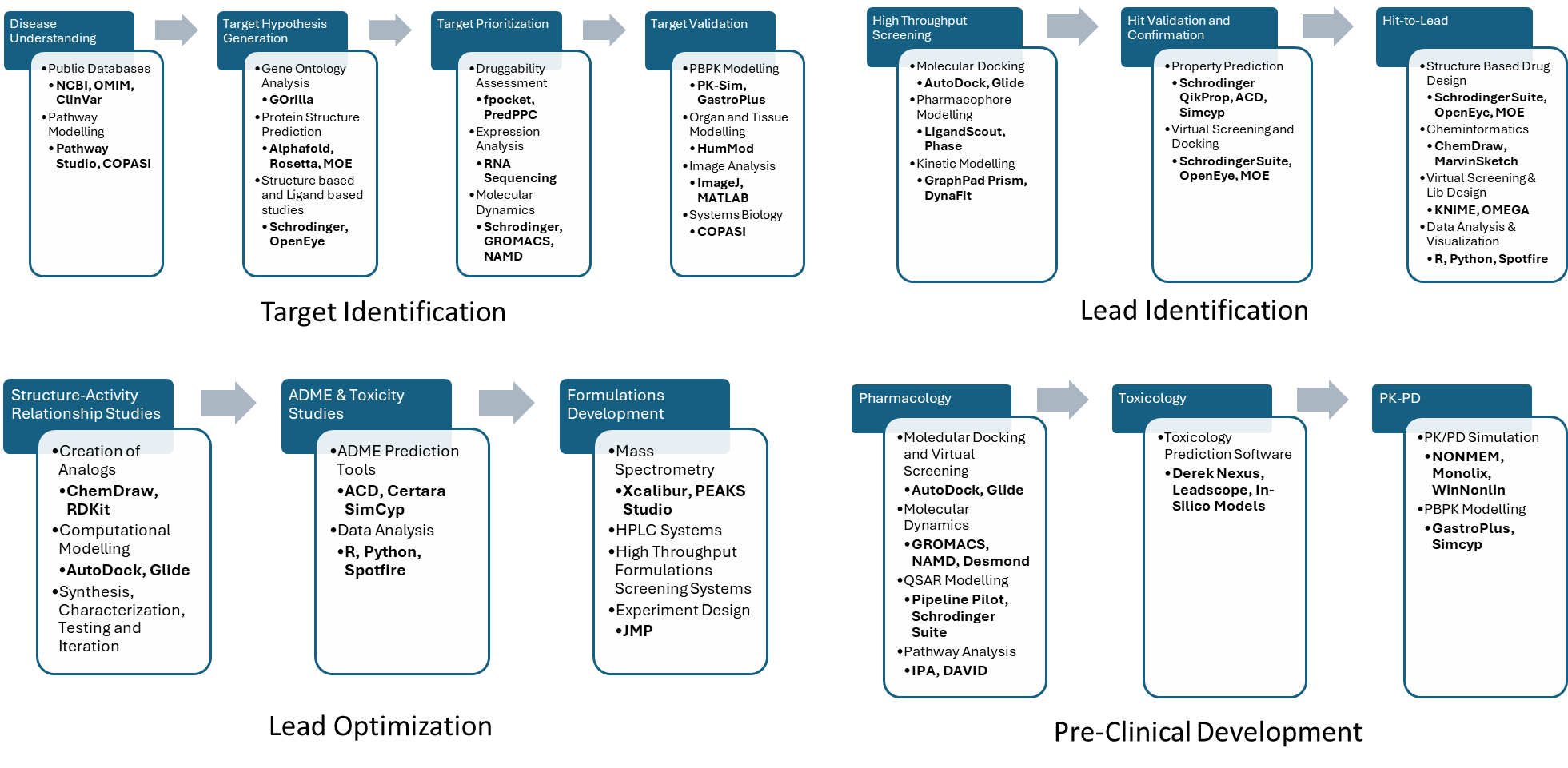
Fig: Different M&S tools used in different phases of drug discovery
Limitations of fragmented scientific modeling ecosystems
Today, individual scientific teams often manage their own modeling tools and infrastructure.
While this provides short-term flexibility, it leads to:
- Multiple teams buying the same software licenses (e.g., Schrödinger, NONMEM, CryoSPARC).
- Redundant infrastructure setups, increasing support burden.
- Inconsistent environments, hurting reproducibility.
- Scientists spending time on tool setup instead of science.
A better way: centralized M&S as a scientific service
Rather than building a one-size-fits-all platform, organizations can deliver Modeling & Simulation as a centralized service, operated by a team with both scientific domain knowledge and technology expertise.
This model gives research teams easy access to validated tools, standardized environments, compute resources and automation workflows, without each team needing to reinvent the wheel.
Implementing M&S as a centralized, shared service offers clear advantages:
- Up to 40% reduction in IT and licensing costs through pooled tools and infrastructure.
- Faster onboarding of scientists with preconfigured, ready-to-use environments.
- Standardization and reproducibility across teams and studies.
- Less time managing environments, more time generating insights.
- A scalable, future-ready foundation for model-informed drug development.
This model doesn’t restrict flexibility, it amplifies it by making high-quality environments available faster, cheaper and more consistently.
What should this service include?
A strong M&S service model includes:
- Provisioning of validated, domain-specific tools (e.g., for QSP, PK/PD, structure-based design).
- Scalable HPC/cloud compute with workflow orchestration.
- Containerized or modular tool delivery to support flexibility.
- Usage tracking for cost visibility and license optimization.
- Centralized knowledge sharing, model libraries, and scientific support.
By centralizing Modeling & Simulation services, not just infrastructure but also the people and processes behind them, pharma companies can reduce operational complexity, unlock cost savings and give their scientists what they need most: the freedom to explore, iterate and discover.
Smarter science. Faster insight. Lower cost. That’s the promise of a centralized M&S service model and it’s well within reach.