In the dynamic realm of laboratory management, staying current with technological advancements is key to ensuring efficiency, compliance and optimal performance. Upgrading your Laboratory Information Management System (LIMS) from one version to another (preferably the latest released and stable version) is a strategic move that requires careful planning and execution.
This article describes Zifo's industry best practices for planning the upgrade of an existing LIMS effectively.
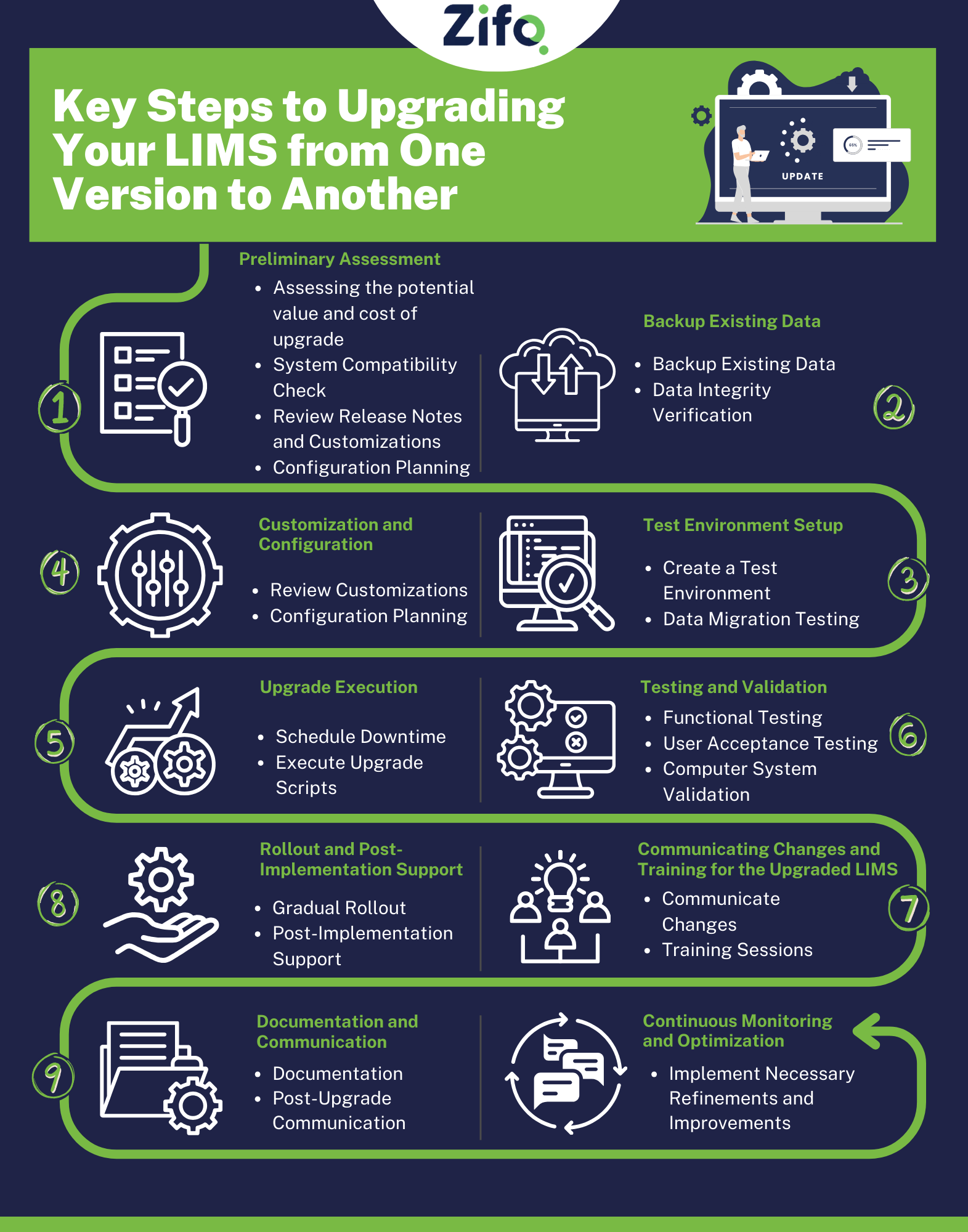
Step 1: Preliminary Assessment
1.1 Assessing the Potential Value of Upgrading LIMS:
The first step to upgrading a LIMS is to understand the reasons behind the upgrade. Upgrading a LIMS offers significant benefits, including enhanced data management and accuracy, improved compliance and reporting capabilities, increased efficiency and productivity through automation, scalability and flexibility to adapt to changing needs, and advanced security features to protect sensitive data. These improvements collectively ensure a more streamlined, compliant, and secure laboratory environment.
1.2 Assessing the Potential Costs of Upgrading LIMS:
When estimating the costs of upgrading a LIMS, organizations should consider several key parameters. These include the initial software and licensing costs, implementation and integration expenses, and training and support costs. Regular maintenance and future upgrade expenses, as well as potential downtime and transition costs, are also important. By evaluating these parameters, an organization can develop a comprehensive estimation of potential value versus potential costs for the LIMS upgrade.
1.3 Review Release Notes:
Before initiating the upgrade process, it's crucial to examine the release notes provided by the LIMS vendor for the new version thoroughly. This phase helps in understanding the updates, improvements, bug fixes, and new features in the latest version. Often, upgrades may involve skipping several versions and jumping directly to a newer one, rather than following a sequential version-to-version path. Having a clear understanding of these updates allows you to anticipate how they might impact your laboratory operations and workflows.
1.4 System Compatibility Check:
After reviewing the release notes, the next step is to verify the compatibility of the new LIMS version with your existing infrastructure. This includes assessing compatibility with hardware, operating systems, databases, web application hosting solutions, and any integrated instruments or systems. Additionally, consider the impact on local desktop clients, which are commonly used in many LIMS applications instead of web versions. Ensuring compatibility upfront helps prevent issues or system failures during or after the upgrade process, minimizing downtime and disruptions to laboratory operations.
1.5 Review Customizations:
Many laboratories customize their LIMS to suit their specific needs and workflows. It's crucial to evaluate any customizations in the current LIMS version and check their compatibility with the new version before upgrading. This involves identifying custom code, scripts, or configurations and determining whether they need to be modified or updated for the latest version of LIMS. Additionally, consider whether existing customizations should remain as-is or be replaced with new, out-of-the-box (OOB) features offered by the upgraded version. If opting for the latter, ensure that information from old customizations can be integrated seamlessly or migrated to maintain continuity and functionality.
1.6 Configuration Planning:
Collaboration between system administrators, end-users and stakeholders is crucial for planning the configuration of the new LIMS version. This step involves identifying user requirements, workflow preferences and any changes needed to adapt the latest version to the laboratory's specific needs. Planning configuration changes ahead of time ensures a seamless transition and minimizes operational disruptions.
Step 2: Backup Existing Data
2.1 Backup Existing Data:
Data is the lifeblood of any laboratory, and ensuring its integrity and safety is paramount during an upgrade. Prior to upgrading, creating a thorough backup of all LIMS data, configurations and user settings is vital. This backup serves as a safety net in case of data loss or corruption during the upgrade process, allowing you to restore your system to its pre-upgrade state if needed.
Step 3: Configuration and Customization
3.1 Restore Customizations:
After backing up the data, the next step is to restore any necessary customizations based on the previous version. This involves implementing custom code, scripts, or configurations identified during the preliminary assessment. Verify that these customizations are compatible with the new version and function correctly.
3.2 Implement Configuration Changes:
Based on configuration planning, apply the identified configuration changes to the new LIMS version. This step involves configuring the system to match user requirements and workflow preferences, ensuring the latest version meets the laboratory's specific needs.
Step 4: Test Environment Setup
4.1 Create a Test Environment:
Setting up a controlled test environment is essential for simulating the LIMS upgrade process and identifying potential issues before implementing the upgrade in a production environment. The test environment should closely mimic your production environment, including hardware, software, and configurations, to allow for thorough testing without affecting live operations.
4.2 Data Migration Testing:
In the test environment, it's crucial to conduct comprehensive testing of data migration processes, where applicable, to ensure a smooth transition of existing data to the new version of LIMS. This involves migrating sample data from the existing system to the latest version and verifying that all data is transferred accurately and completely. Testing data migration in a controlled setting helps identify and resolve issues before the upgrade, reducing the risk of data loss or corruption.
Step 5: Upgrade Execution
5.1 Schedule Downtime:
Planning for downtime is essential to minimize disruptions during the upgrade process. Schedule the upgrade when laboratory operations are least affected, such as during off-hours, site closures or weekends. Communicating the downtime schedule to all stakeholders and end-users helps manage expectations and minimizes inconvenience caused by the upgrade.
5.2 Execute the Upgrade:
Upgrade scripts typically include instructions for updating the database schema, modifying application code, and implementing any other changes required for the new version. Carefully following the upgrade scripts ensures that the process is executed correctly and efficiently, reducing the risk of errors or complications. Additionally, it is critical to identify a rollback or exit strategy in case of unexpected errors or failures that cannot be resolved immediately. This strategy provides a contingency plan to revert the system to its pre-upgrade state, minimizing disruptions to laboratory operations.
Step 6: Testing and Validation
6.1 Functional Testing:
After completing the upgrade, conduct comprehensive functional testing to ensure all LIMS features and functionalities work as expected. This involves testing various modules, workflows, and user interactions to identify any issues introduced during the upgrade process. Functional testing ensures the upgraded LIMS meets the requirements and expectations of end-users and stakeholders.
6.2 User Acceptance Testing (UAT):
Engaging end-users in user acceptance testing (UAT) ensures the upgraded LIMS meets their needs and expectations. End-users should be allowed to test the system in a real-world environment and provide feedback on usability, performance, and functionality. Incorporating user feedback during UAT helps identify areas for improvement and ensures the upgraded LIMS meets the needs of its primary users.
6.3 Computer System Validation (CSV):
Performing Computer System Validation (CSV) is essential to ensure the upgraded LIMS complies with regulatory requirements and standards. A phased approach is recommended, starting with full validation in the test environment, followed by minimal UAT or Performance Qualification (PQ) and smoke testing in the production environment.
Since many tests cannot be performed in the live production environment, completing validation in the test environment helps identify issues while ensuring production stability. This strategy allows for thorough testing while minimizing disruptions to laboratory operations.
If the system is already validated, it is also crucial to evaluate existing validation documentation, including functional specifications, design specifications, system architecture, risk assessments, Installation Qualification (IQ), Operational Qualification (OQ), change management processes, and backup/recovery procedures. Review and update these artifacts to ensure comprehensive validation and compliance.
Step 7: Communicating Changes and Training for the Upgraded LIMS
7.1 Communicate Changes:
Before rolling out the upgraded LIMS, it's essential to communicate any changes or new features introduced in the latest version to all stakeholders and end-users. This helps manage expectations and prepares users for necessary adjustments to their workflows or processes.
7.2 Training Sessions:
Conducting training sessions before the Go-Live for end-users is essential to ensure a smooth transition to the upgraded LIMS. Training should cover new features, workflow changes, and any other updates relevant to end-users' roles and responsibilities. Providing comprehensive training helps users adapt to the changes more quickly and reduces the risk of errors or confusion during the transition.
Step 8: Rollout and Post-Implementation Support
8.1 Gradual Rollout:
A gradual rollout can be beneficial during initial system implementation, allowing end-users to adjust over time. However, this approach may not always be feasible for upgrades, especially those with complex integrations. Many customers prefer a full system upgrade over a phased approach to avoid potential integration issues. Although a phased rollout may not always be practical for upgrades, consulting Subject Matter Experts (SMEs) is crucial to determine the best approach based on the system's complexity and operational requirements.
8.2 Post-Implementation (Hypercare) Support:
Establishing a support system for post-implementation issues ensures a smooth transition to the upgraded LIMS. This may include providing a helpdesk or support hotline for users to report problems or ask questions and assigning dedicated support staff to address technical or operational challenges. Prompt and effective post-implementation support minimizes downtime and helps ensure users resolve issues quickly.
Step 9: Documentation and Communication
9.1 Documentation:
Documenting the entire upgrade process is crucial for future reference and troubleshooting. This includes configurations, customizations, upgrade procedures, and user manuals. Comprehensive documentation ensures that relevant information is readily available for ongoing maintenance and troubleshooting of the upgraded LIMS.
9.2 Post-Upgrade Communication:
Effectively communicating the successful upgrade to stakeholders and end-users is essential for fostering transparency and accountability. This includes notifying them of the upgrade’s completion, providing guidance on accessing new features, and addressing common queries or concerns. Clear and timely communication ensures all stakeholders reamin informed and engaged throughout the upgrade process.
Step 10: Continuous Monitoring and Optimization
10.1 Implement Necessary Refinements and Improvements:
Continuous improvement involves enhancing the technical aspects of the LIMS and leveraging captured data for data-driven decision-making to optimize system performance, data management, and user experience. This approach identifies opportunities for improvement in both business and laboratory processes, ensuring the LIMS evolves to meet changing needs. Additionally, incorporating data management principles and FAIR data (Findable, Accessible, Interoperable, and Reusable) increases the value and accessibility of laboratory data, supporting more effective analysis and decision-making.
Conclusion:
Upgrading your LIMS from one version to another is a strategic investment in the efficiency and longevity of your laboratory operations. By following the foundational steps outlined in this guide, laboratories can navigate the intricacies of the upgrade process, ensuring a seamless transition and reaping the benefits of the enhanced features and functionalities offered by the latest LIMS version.
If you have any questions or would like to discuss LIMS upgrades further, please get in touch at info@zifornd.com.


