What comes to mind when you think of the term 'Clinical trial’?
Pretty sure it's a large hospital site having all the subjects enrolled in the study, physicians interacting with the subject, and lots of things going on in the background. So, what if this hospital – site centric is called the traditional approach? And what does the future hold for clinical trials without a site? That’s what a “decentralized” clinical trial is all about - a clinical trial with no site. In 2011, the first ever Decentralized Clinical Trial (DCT) was initiated, and today’s biometrics technology has evolved to support remote subject data collection. It is well known that retaining subjects through a trial is more demanding than getting subjects to enrol in the first place. So, What matters in a clinical trial is the subject's comfort.
Speed Up recruitment, Ensure retention. Drive greater engagement
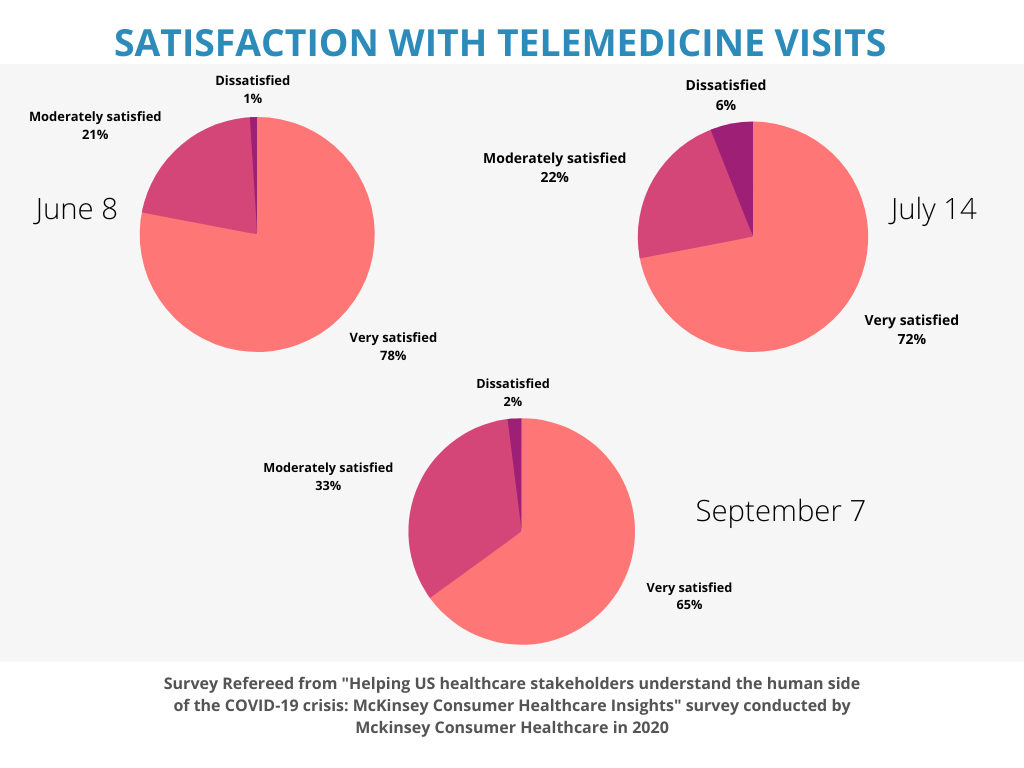
The COVID-19 pandemic has significantly influenced the growth of decentralized clinical trials. As the pandemic intensified, patient access to clinical sites became very limited due to travel restrictions. And since more than 70 % of the potential participants lived more than two hours away this caused a big problem. Surveys1 show that between the months of January 2020 and April 2020, the number of monthly trials reduced by 50 %. To keep the trials going, sponsors had to find an alternate solution, and that was “telemedicine”. Looking back at the pre covid era, we can see that to a certain degree, decentralized clinical trials had existed before. The pandemic has accelerated the increase in numbers of decentralized clinical trials. In a survey2 conducted in 2020, up to 98 % of the clinical trial subjects reported satisfaction with telemedicine, and 72 % of the physicians favoured remote engagements in comparison with in-person visits.
DCT VS HYBRID
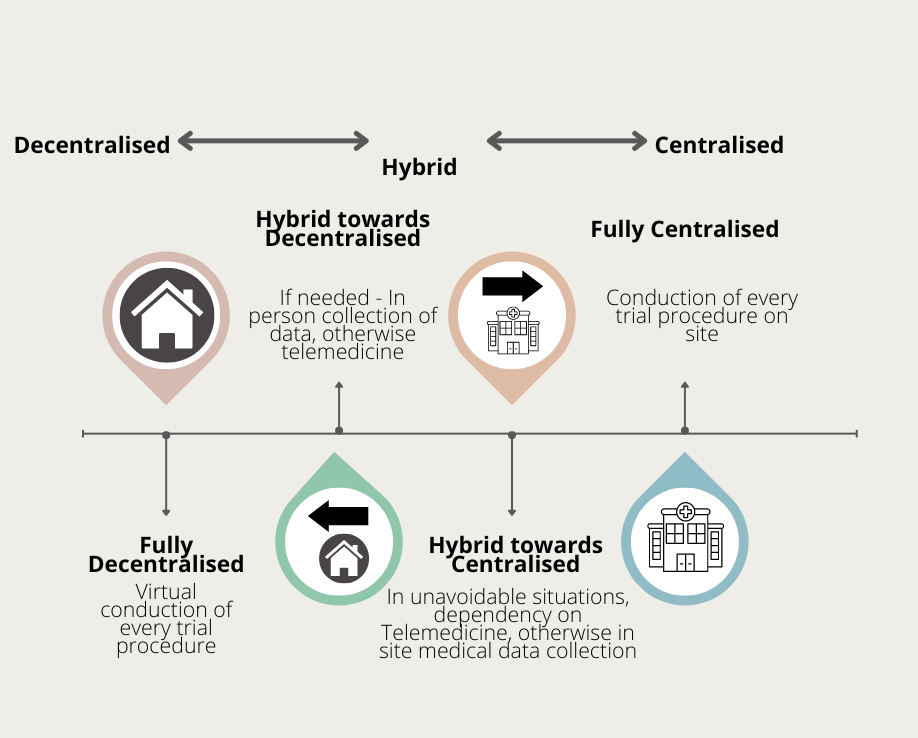
Even though decentralised clinical trials sounds theoretically reliable, it is not suitable for all types of clinical trials. A hybrid approach that brings together the best of both centralised and decentralised clinical trials is then the best approach. In a hybrid trial, technology is utilized wherever possible to reduce the need for in-person site visits. For instance, smartwatches can record their vitals periodically and notify the site – meaning the patient does not travel to the site for this routine event. So we see, to achieve decentralised clinical trials, a platform for the exchange of information is mandatory.In addition to the smartwatches mentioned - devices such as blood glucose monitors, blood pressure monitors, pedometers (which displays number of steps walked and the the calories burned propotional to that activity) will aid as a medium for the exchange of information.
Challenges
Regardless of the benefits associated with DCTs, there are challenges. For CROs, the top 3 challenges stated are the effectiveness of study execution at the patient’s residence, clear regulatory guidance, and the ability to integrate data into the existing clinical trial data management (CTDM) ecosystems.
Other pressing challenges are,
- Drug distribution and management must be done with utmost care, security and regulatory compliance.
- The use of technology requires identifying modern technology vendors adhering to study compliance.
- 1. Technology vendors should be available always to provide guidance and solve the technical issues faced.
- 2. For cases where patients might not be aware of using technical devices, healthcare professionals or caregivers should be appointed and trained.
- Caregivers should be trained properly about administering study drugs or tests to patients, especially for persons with severe health conditions or the elderly.
- 1. Since the data is collected electronically, there should be multiple layers of security followed to avoid data breaches.
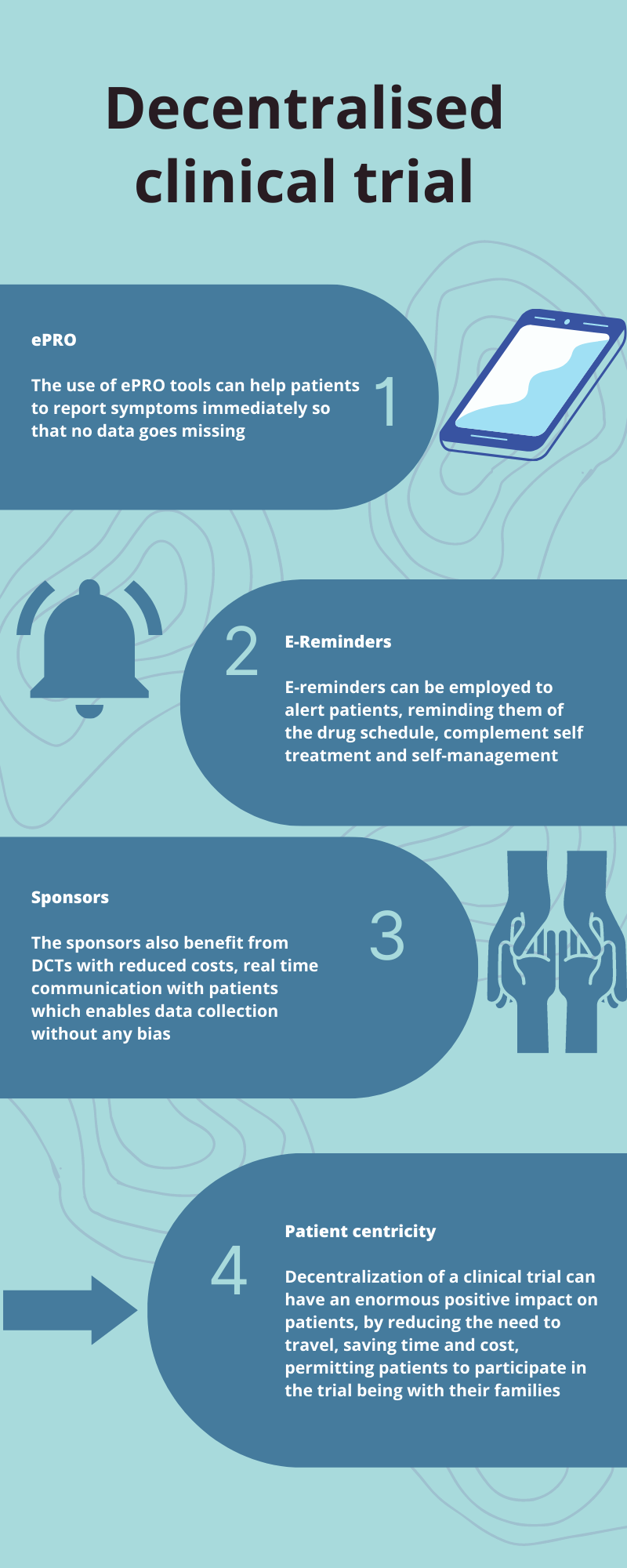
Despite these challenges, DCTs are on their way to becoming part of the future of drug discovery and development assisted by modern technology.
Conclusion
DCT is a rapidly growing field and data from EY-Parthenon3 of 69 sponsors and contract research organisation (CRO) decision makers indicates that 50 % of the clinical trials will be hybrid or decentralised by 2024. The Covid-19 pandemic and it’s after effects has demonstrated that DCTs are critical and effective. History often shows us that technology and innovation often grows exponentially when a major need is present - -and in the COVID 19 case this was the case – “adversity is the mother of innovation” a quote attribute to Einsten. It seems that further adoption of a DCT approach will be driven by regulatory approval/guidance and advancements in data integration. Many CROs and CTDM vendors across the globe are taking the necessary steps to fill in the gaps.
References:
1. Clinical trial recovery from COVID-19 disruption
3. How biopharma and CROs can create value in a shift to decentralized trials