
Assay development and validation, instrument data acquisition and integration, regulatory and QC data reporting, lab inventory and metrology, workflow automation and validation

Manipulation of biology at the 'Omic level and its downstream effects uses many different techniques such cloning, plasmid design, cell transfection and protein/product production.
Molecular mechanics and molecular dynamics, virtual screening, structure-based design, docking & QSAR, high-performance computing, storage and cloud informatics

PLM, recipe and ingredients management in ERP, formulation development process and statistical DOE and analytical data collection and management in LIMS and ELN

Design of experiments (DoE), inventory & materials management, robotics, instrument integration, workflow automation, system monitoring and data analytics
Early Pharmacology, ADME, Assay Automation, PK/PD, Assay Development & Validation, Bioanalysis, Inventory Management etc. and the linked regulatory aspects of GLP and IND/NDA/BLA creation support.

"Always on", end-user support, proven scale, quality, processes and knowledge of scientific applications have a measurable impact on end-user efficiency, satisfaction and application downtime.

16s/ITS Metagenomics & Meta-transcriptomics, pre-processing of raw data, dereplication of sequences and chimeric reads removal, OTU picking, Quality filtering, taxonomical classification
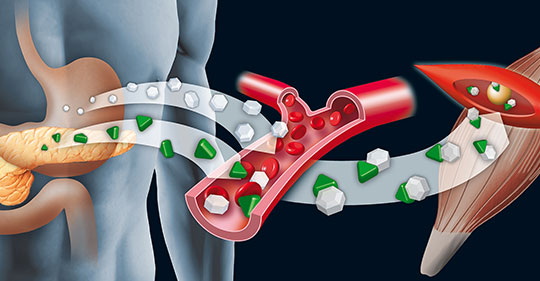
Pharmacology, ADME cascade automation, PK study design, resource management, data acquisition, Bioanalysis, PK modelling and regulatory submissions

Bidirectional translation of biomedical knowledge from the early research laboratory, through development and manufacturing to clinical & patient-oriented and population-based research



